Our Research Targets: “Receptors”
There are many “receptors” on the surface, inside, and in the nucleus of cells. Ligands such as hormones that bind specifically to these receptors regulate the transmission of information through these receptors. We are conducting research on receptor chemistry, especially on nuclear receptors that regulate gene transcription in the cell nucleus and neuropeptides and their receptors that are involved in pain and analgesia, and on the molecular recognition and activation mechanisms of receptors/ligands.
Environmental Science: Structure-function relationship between environmental chemicals and receptors
We enjoy a convenient life due to the development of civilization. As a result, there are a variety of chemicals in the environment. When these chemicals affect us, the first step in many cases is the binding of the chemical to the receptor. We are conducting structure-function relationship analysis of nuclear receptors in the nucleus of cells by means of binding assays and transcriptional activity measurements.
In humans, there are 48 types of nuclear receptors. Among them, we discovered the estrogen-related receptor gamma type, to which bisphenol A binds very strongly. Furthermore, we have conducted groundbreaking research that has attracted social attention, such as the discovery of a new generation of halogen-containing bisphenol (bisphenol AF) that exhibits unique estrogen receptor response characteristics. Recently, these results have led to the development and expansion of important research that will lead to the elucidation of the molecular mechanisms of transcriptional regulation.
~The Story of the Discovery of Bisphenol AF Bonding~
“You are doing a lot of experiments for nothing.” That is what they told me, but I could not give up. Large-scale research had been conducted on the problem of endocrine disruptors, or so-called environmental hormones, and it had been reported that the binding of bisphenol A to estrogen receptors was 1,000 to 10,000 times weaker than that of natural hormones. Furthermore, even at that time, more than 1,000 compounds had already been screened (i.e., many were analyzed to select the target compounds). And it was reported that the binding ability of compounds called endocrine disruptors to estrogen receptors was generally weak. Therefore, attention in the laboratory had shifted to the existence of “non-estrogen receptor” nuclear receptors to which bisphenol A binds. Therefore, binding tests of more than 200 derivatives of bisphenol A to various nuclear receptors were conducted every day. I felt like a robot. In this context, everyone was aware that bisphenols bind to estrogen receptors only weakly, to the point where it could be said that they hardly bind at all.
However, having experience in chemically synthesizing halogenated phenylalanines, incorporating them into peptides, and measuring their biological activity, it seemed to me that derivatives of bisphenol A with two phenolic skeletons were the preferred skeleton for receptor binding (previolated structure). That is why I continued silently to test the binding of about 200+ derivatives of bisphenol A to estrogen receptors, which no one had ever done before. Of course, most compounds barely bound. One day, however, while I was sitting in a dimly lit and unpopular radioisotope (RI) center, watching the results of the radiation counts being written out one after another, hoping that they would be finished soon, I noticed a column where the values were much lower than those of other compounds.
Thus, I found new-generation bisphenol containing halogen, such as bisphenol AF. I am grateful to my professor at the time for his tacit approval of this seemingly futile experiment. As any experimental scientist would think, “You never know the results of an experiment until you try it. The result of an experiment is not known until it is tried.
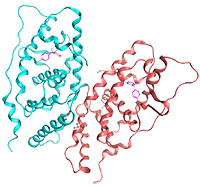
X-ray crystallographic analysis of the binding of bisphenol A to the nuclear receptor ERRγ. We discovered the nuclear receptor ERRγ, which binds very strongly to bisphenol A, an endocrine disruptor reported to have adverse effects on the cranial nervous system and reproductive system, and clarified its binding structure for the first time in the world.
Receptor Chemistry: Structure-function analysis of nuclear and neuropeptide receptors
Nuclear receptors are transcription factors that precisely regulate transcription in the cell nucleus. And the receptor for the female hormone estrogen is also a nuclear receptor. And surprisingly, the two types of estrogen receptors, alpha and beta types, exist as completely different genes. The exact reason why the two types of receptors exist is not clear. We are developing research that will help us to solve this mystery.
Furthermore, we are studying peptide hormones such as analgesic peptides in the brain, pain nerve stimulating peptides, and thrombin receptor built-in ligand peptides in the blood coagulation system. We are engaged in research to elucidate at the molecular level where and how peptides bind to and are activated by receptors. We design and synthesize amino acids with specific structures, synthesize peptides, create genetically engineered modified receptors, conduct receptor binding studies, perform bioactivity assays, analyze the conformation of peptides (NMR, CD), perform molecular modeling by computer, and analyze conformational changes. Recently, we have been studying novel chemical modification sites of receptors.
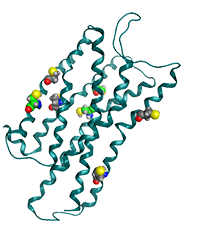
Structure of the pain peptide, nociceptin receptor. The nociceptin receptor is a G-protein coupled receptor located on the cell membrane surface. This figure shows a CPK model of the seven cysteine residues in the transmembrane region of the receptor (PDB ID: 4EA3).
Brain Neurochemistry: Analysis of Chemical Exposure Effects on Behavior
In particular, we are analyzing the effects of a bisphenol A diet on the body using the activity rhythms of mice and other experimental animals as indices. To elucidate the molecular mechanisms that influence activity rhythms, we are conducting genetic analysis of clock genes, clock proteins, and neurotransmitter peptides involved in circadian rhythms.