Nowadays, enantioselective hydrogenations with asymmetric catalysis are regarded as a convenient tool for preparing optically active compounds. The asymmetric hydrogenations of alkenes, ketones and imines had intensively been studied and applied to lab-scale and industrial synthesis of useful compounds by many chemists. Meanwhile, heteroarenes, such as pyridines and pyrroles, had been formidable substrates for the asymmetric hydrogenation, because their unsaturated bonds are highly stabilized with their own aromaticity. Because of the stabilization, the dearomative reduction requires harsh conditions, which are disadvantageous for high degree of stereochemical control. However, the highly stereo-controlled dearomative hydrogenation may offer a powerful method for constructing 5- or 6-membered chiral heterocyclic skeletons, which are ubiquitously seen in many biologically active compounds.
We reported a highly enantioselective hydrogenation of indoles 1 with a chiral rhodium catalyst bearing a chiral ligand, PhTRAP L1, in 2000.1) This is the first success of highly enantioselective hydrogenation of 5-membered heteroarenes. Furthermore, we found that the ruthenium complex also worked as a highly enantioselective catalyst for the hydrogenation of indoles.2) Pyrroles 2,3) imidazoles 3, oxazoles 4,4) and azaindoles 55) were converted into the corresponding chiral heterocycles with high enantioselectivities through the chiral ruthenium catalysis. In the hydrogenation of 5, the 5-membered heteroarenes were exclusively saturated with H2 to give the azaindolines in high yields.
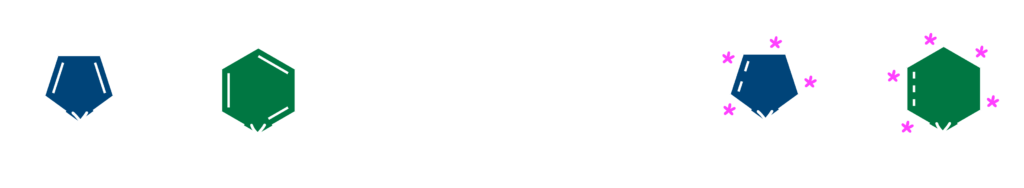
Our group made first success in the catalytic asymmetric hydrogenations of pyrimidines6) and isoxazoles.7) Although the L1–ruthenium catalyst failed to provide the desired products, the highly enantioselective hydrogenations of these heteroarenes were successfully developed by using chiral iridium complexes. A broad range of 2,4-disubstituted pyrimidines 6 were transformed into 1,4,5,6-tetrahydropyrimidines with over 90% ees through the chiral iridium catalyst bearing a Josiphos-type ligand L2. Meanwhile, optically active L3–iridium complex is effective for the asymmetric reduction of 2,3,5-trisubstituted isoxazolium salts 7 to give the chiral isoxazolines or isoxazolidines with up to 90% ee. It is noteworthy that the N–O bond remains intact during the reductive process with transition-metal catalyst.

Now, we are studying on the asymmetric hydrogenation of other heteroarenes. Concurrently, we are trying to construct new chiral catalyst systems for asymmetric heteroarene hydrogenations.
1) J. Am. Chem. Soc. 2000, 122, 7614[link to journal article]; Org. Lett. 2004, 6, 2213.[link to journal article]; Tetrahedron: Asymmetry 2006, 17, 521.[link to journal article]
2) Org. Lett. 2006, 8, 2653.[link to journal article]
3) J. Am. Chem. Soc. 2008, 130, 808.[link to journal article]
4) J. Am. Chem. Soc. 2011, 133, 7312.[link to journal article]
5) Angew. Chem. Int. Ed. 2016, 55, 11859.[link to journal article]
6) Angew. Chem. Int. Ed. 2015, 54, 2393.[link to journal article]
7) Chem. Eur. J. 2016, 22, 8610.[link to journal article]
