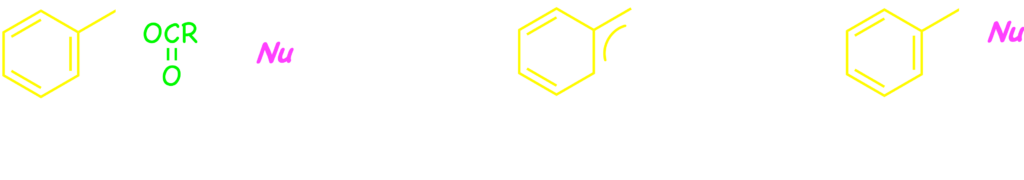
Nucleophilic substitution is one of fundamental reactions in organic chemistry. In the reaction, alkyl bromides, iodides, and sulfonates are mostly employed as the electrophilic substrates. Commonly, carboxylate and hydroxy functionalities are inert for the leaving group.
Allylic carboxylates 1 work as good electrophile for the nucleophilic substitution in the presence of palladium catalyst. The palladium-catalyzed allylic substitution, Tsuji-Trost reaction, is known to proceed through electrophilic (pi-allyl)palladium intermediate A, which is generated from the oxidative addition of the ester to palladium(0) species. The catalytic reaction widely used for not only constructing molecular frameworks but also the removal of allyl protection.
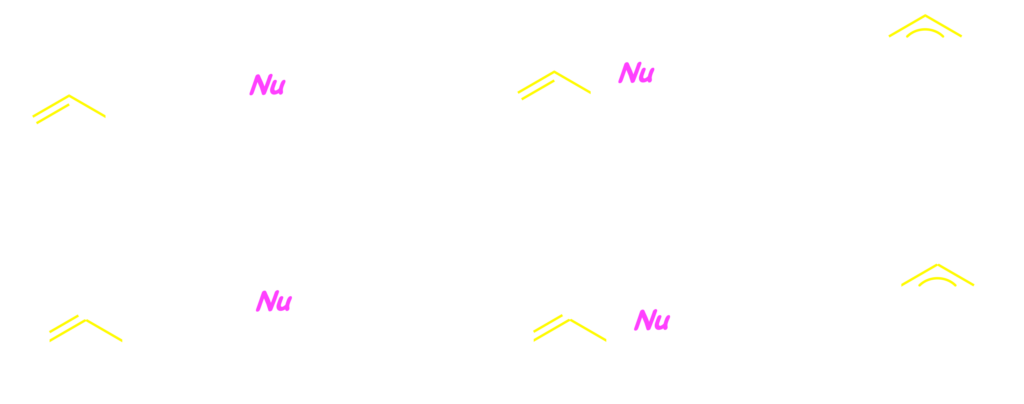
Meanwhile, benzyl compounds usually exhibit similar reactivity to the corresponding allyl ones, because both groups is constituted of an sp3 carbon bonding to unsaturated C–C group. We had successfully developed the benzylic substitution of benzyl carbonates and acetates 2 with stabilized carbanions and amines through palladium catalysis.1) Since then, we reported that a broad range of nucleophiles were applicable to the palladium-catalyzed benzylic substitution.2) In the catalytic reaction, the palladium(0) species cleaves the bezylic C–O bond to form (pi-benzyl)palladium, which undergoes the attack of the nucleophile.3)
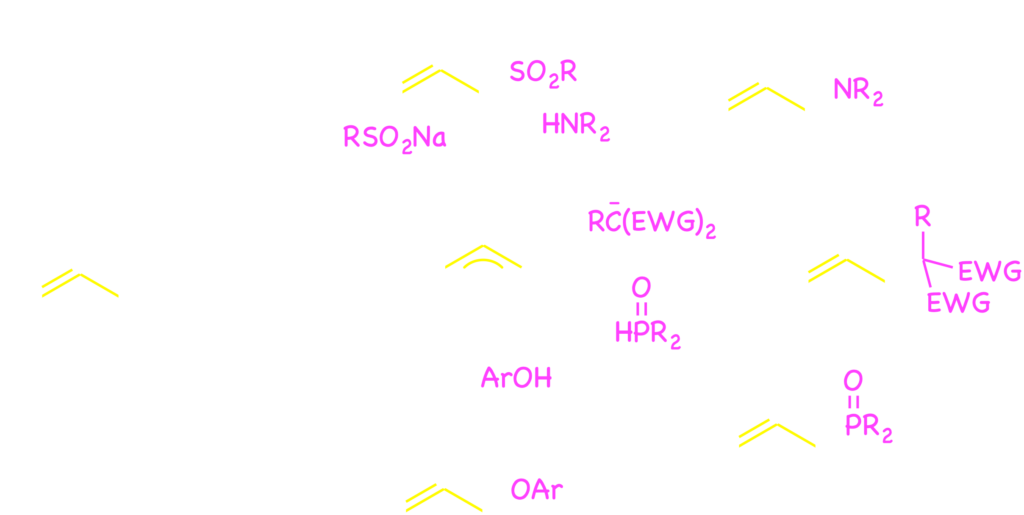
Organometallic compounds are usable as the nucleophiles for the palladium catalysis involving the benzylic C–O bond cleavage. The benzylic carbonates and acetates 2 were coupled with arylboronic acids 34) and arylstannans5) through the palladium catalysis. As a related reaction, we reported a decarboxylative C–C bond formation of benzyl fluorobenzoates 4.6)
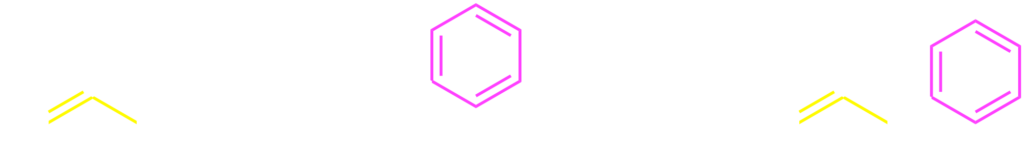
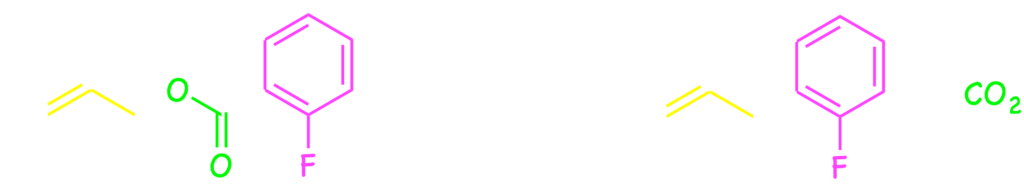
Furthermore, some new types of catalytic cyclization have been developed by means of the palladium catalysis and felicitous design of substrate. Formal [4+2] cycloadditions of o-quinodimethane were developed with the catalysis by installing (silly)methyl group at the o-position of benzyl carbonates.7) Furthermore, intramolecular SN‘-type aromatic substitution was developed by connecting a nucleophile with the m-carbon through C3 tether.8)
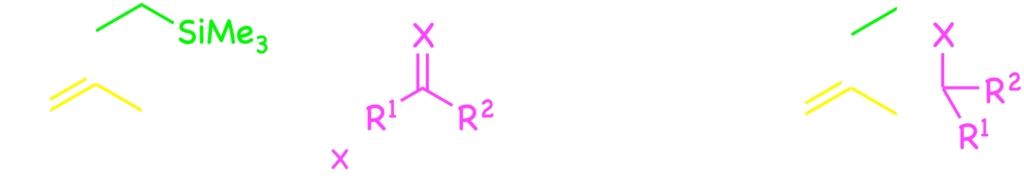
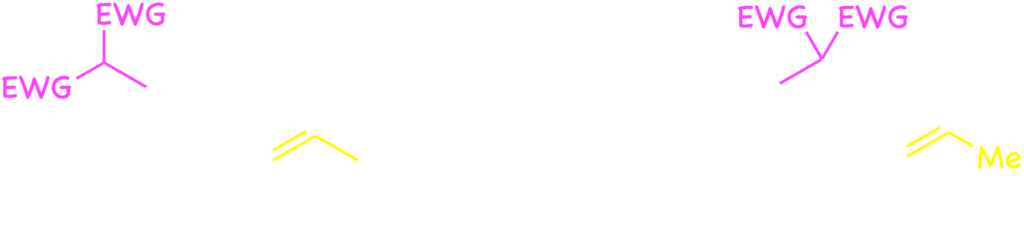
Now, we continue to study on the catalytic reactions involving the cleavage of the benzylic C–O and related bonds with transition-metal complexes.
1) J. Am. Chem. Soc. 2003, 125, 12104[link to journal article]; Org. Lett. 2004, 6, 3545[link to journal article]; Tetrahedron Lett. 2007, 48, 6109.[link to journal article]
2) Org. Lett. 2005, 7, 2973[link to journal article]; Org. Lett. 2008, 10, 1979[link to journal article]; Chem. Lett. 2017, 46, 1814.[link to journal article]
3) Org. Process Res. Dev. 2019, 23, 1568.[link to journal article]
4) Org. Lett. 2005, 7, 945[link to journal article]; Chem. Commun. 2005, 5899[link to journal article]; Heterocycles 2007, 74, 233[link to journal article]; Org. Lett. 2008, 10, 973.[link to journal article]
5) Chem. Lett. 2008, 37, 796.[link to journal article]
6) Synlett 2017, 28, 2573.[link to journal article]
7) J. Am. Chem. Soc. 2007, 129, 3802[link to journal article]; J. Am. Chem. Soc. 2009, 131, 12904[link to journal article]; Org. Lett., 12 (19), 4332–4334 (2010).[link to journal article]
8) Org. Lett., 14 (1), 338–341 (2012).[link to journal article]
